Edward Fishman: The Collapse of the Oil Empire?
EPISODE SUMMARY Podcast host Alex Gabuev is joined by Edward Fishman, a senior research scholar at the Center on Global Energy Policy at Columbia University,…
Thought Leader: Edward Fishman
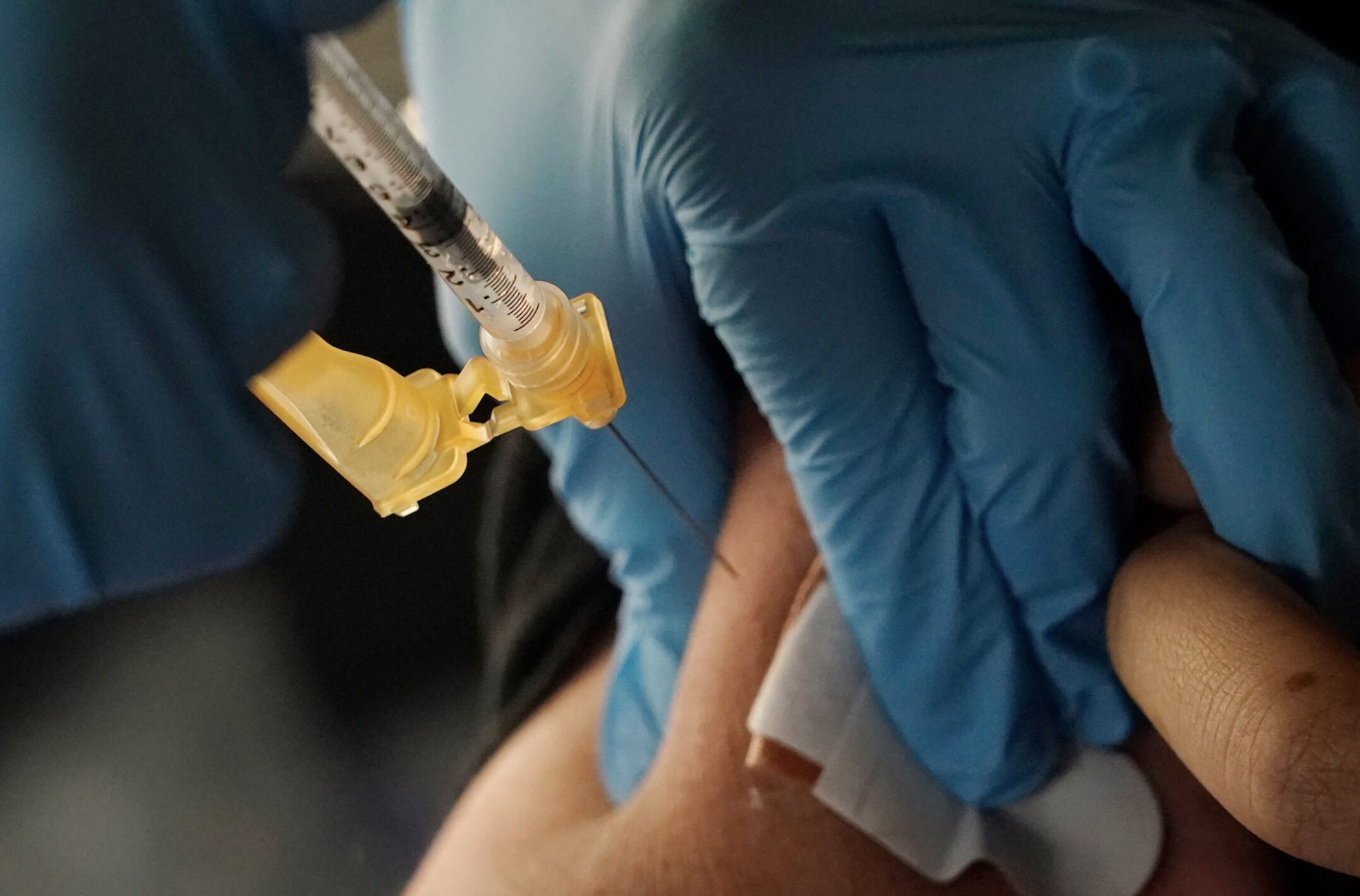
Washington — Former Food and Drug Administration (FDA) Commissioner Dr. Scott Gottlieb predicted Sunday that the agency he helmed will authorize Pfizer’s coronavirus vaccine for emergency use in children ages 5 to 11 by the end of October.
In an interview with “Face the Nation,” Gottlieb, who serves on Pfizer’s board of directors, said the drug company is expecting to have data on its vaccines in young children before the end of September, which will then be filed with the FDA “very quickly.” The agency then has said it will be weeks, rather than months, before determining whether it will authorize the vaccine for kids ages 5 to 11.
“In a best-case scenario, given that timeline they’ve just laid out, you could potentially have a vaccine available to children aged 5 to 11 by Halloween,” Gottlieb said. “If everything goes well, the Pfizer data package is in order, and FDA ultimately makes a positive determination, I have confidence in Pfizer in terms of the data that they’ve collected. But this is really up to the Food and Drug Administration to make an objective determination.”
Pfizer has been conducting clinical trials of its two-dose vaccine in children 2 years and older, and its approval could be crucial to helping combat the spread of the highly contagious Delta variant in schools. Children represent 25% of new COVID-19 infections.
The shot has already been authorized for children ages 12 to 15, and Gottlieb said he believes COVID vaccines will eventually be among those required for children in public schools.
“I think you’re going to see more local school districts and governors make those recommendations,” he said. “Eventually ACIP is going to make a recommendation about whether this should be included in the childhood immunization schedule. My guess is they’re waiting for more of the vaccines to be fully licensed to make that kind of a recommendation. But I would expect this eventually to be required as part of the childhood immunization schedule.”
For parents who may be wary of their children receiving a vaccine that is under emergency use, rather than fully approved by the FDA for children, Gottlieb encouraged them to consult with their pediatricians, but stressed they are not facing a “binary decision” of getting their children vaccinated against COVID-19 or not.
“There’s different ways to approach vaccination. You could go with one dose for now. You could potentially wait for the lower dose vaccine to be available, and some pediatricians may make that judgment. If your child’s already had COVID, one dose may be sufficient. You could space the doses out more,” he said. “So, there’s a lot of discretion that pediatricians can exercise, making largely off-label judgments, but exercising discretion within the context of what an individual child’s needs are, their risk is, and what the parents’ concerns are.”
While the FDA is expected to make a decision on whether to authorize COVID vaccines in children in the coming weeks, federal health agencies are also weighing whether to approve booster shots for vaccinated Americans.
The Biden administration initially announced in August that it was prepared to begin offering the boosters the week of September 20, and Americans would need to get their additional shots eight months after receiving their second vaccine dose. But Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told “Face Nation” last week that it may only be Pfizer’s booster that receives federal approval by September 20.
The FDA’s advisory committee is scheduled to meet Friday to discuss the booster shots, and Gottlieb said the agency is positioned to act “very quickly” depending on the outcome of the meeting. If the boosters are approved, he said a Centers for Disease Control and Prevention advisory committee would then recommend which populations would get them first, likely those who are at higher risk of severe illness or death from COVID-19 such as elderly Americans living in nursing homes.
Pfizer has already filed its application with the FDA for approval of its booster, and Gottlieb predicted Johnson & Johnson will likely be next to do so.
“They have very good data also looking at boosters. They’ve showed a good response,” he said of Johnson & Johnson. “And I think that vaccine also could be in a position to get authorized by FDA in short order.”
Edward Fishman: The Collapse of the Oil Empire?
EPISODE SUMMARY Podcast host Alex Gabuev is joined by Edward Fishman, a senior research scholar at the Center on Global Energy Policy at Columbia University,…
Thought Leader: Edward Fishman
Leana Wen: How long does covid booster protection last?
More evidence highlighting the benefit, and limitations, of covid-19 vaccines. After the Centers for Disease Control and Prevention shifted its coronavirus vaccine guidance from a…
Thought Leader: Leana Wen
Dr. Sanjay Gupta: How Wearable Tech Transforms Health
We’ve come a long way in integrating technology into our daily lives, but could wearable tech actually help you live longer? From detecting heartbeat irregularities to flagging signs of…
Thought Leader: Sanjay Gupta